In the last blog we highlighted some real world limitations in the use of PSG for diagnosing bruxism in clinical dental practice. However, without the benefit of evidence–based PSG research we will still be in the dark.
Dentists generally become involved only after ‘the rubber hits the road’ i.e. when there’s observable occlusal interface and/or other stomatognathic damage (e.g. TM joint dysfunction, masticatory muscle symptomology). Most are blind to what comes before! (Fig. 3) However, the ability to subcategorize bruxism into different phenotypes according to the brain state in which it occurs is extremely important when tailoring treatment for these patients. Moreover, we need to know if we’re, in fact, dealing with secondary bruxism.
Fig. 3
NREM Sleep Bruxism
In NREM sleep, most micro-arousals tend to occur in a structured and repetitive manner known as the cyclic alternating pattern (CAP). SB patients are thought to have a heightened responsiveness to sleep arousals. 88% of NREM SB occurs in CAP phase A, particularly A3[1]. CAP phase A is ‘not the generator of RMMA/SB events but rather provides the permissive window for these motor events to occur during an unstable sleep condition’ [2]. It is important to realise that 60% of normal subjects also exhibit RMMA episodes but at a low frequency of about 1 episode an hour whereas it is 3 times more frequent and EMG levels are 40% higher in SB subjects. Some researchers now believe that NREM SB is likely an extreme manifestation of a complex physiologic oromotor behavior serving homeostatic purposes such as maintenance of oro-esophageal pH and lubrication [3] as well as upper airway patency during sleep [4]. Therefore, in predisposed individuals and those with hyper-arousal from other painful comorbidities, insomnia or fragmented sleep from unresolved Upper Airway Resistance Syndrome (UARS) or Periodic Limb Movement Syndrome (PLMS), we can expect intensification of the RMMA in either frequency and/or amplitude. Fluctuations in central nervous system neurochemicals (e.g. dopamine) and drugs (e.g. clonidine)/conditions that influence sleep architecture by altering REM sleep onset/duration, or autonomic nervous system control may also influence the occurrence and distribution of RMMA during sleep.
Using additional Pes (esophageal pressure) monitoring during PSG, Simmons and Prehn were able to demonstrate that inspiratory flow limitations and respiratory effort arousals associated with UARS triggered tonic NREM SB (i.e. clenching)[5]. They posited that increased masseter muscle activity, by stabilizing the mandible in a forward direction and increasing genioglossus tone, protects the upper airway in situations where there is vulnerability towards airway collapse. More recently, a local retrospective PSG investigation of a large sample (n=147) of Asian adult OSA patients found that 33.3% had concomitant SB, and these patients also demonstrated significantly more respiratory-related arousals and oxygen desaturations than OSA counterparts without SB[6]. The investigators, however, found no evidence that the Apnea-Hypopnea Index(AHI) or Oxygen Desaturation Index(ODI) were associated with the odds of experiencing SB. In contrast to the earlier reported study on UARS, most of the RMMA observed in these OSA-SB patients were phasic in nature. The effectiveness of mandibular advancement splints (MAS) and nasal continuous positive airway pressure(CPAP) therapy in reducing SB has been substantiated in PSG studies by others[7, 8] and offered as proof of concept.
The varying ability to deploy homeostatic NREM SB for airway protection and the reactive sensitivity of the pharyngeal dilators of each individual are influenced by age, genetics, pharyngeal collapsibility, sleep arousal reactivity, presence of autonomic nervous system dysregulation, progesterone levels, craniofacial morphology etc. This probably accounts for the higher prevalence of NREM SB in patients with UARS and mild OSA compared to those with severe OSA, and also explains the divergent relationship between SB and OSA prevalence with age [9].
Take, for instance, the unusually high prevalence of SB reported in children (30% in 5-6yr olds, 14-20% < 11 yrs) and the relatively low prevalence(3%) in individuals over 60 years [9]. We used to reassure worried parents that 1 in 3 children typically stop their nocturnal tooth grinding when they reach their twelfth birthday. The observation was valid although previously we really didn’t know the reason for it! We know now that the human airway only reaches its mature dimension around 12 years whereas adenoid growth begins at 2.5 years and peaks at around 5-6 years. NREM SB in children should therefore alert us that there are ongoing airway patency issues during sleep (e.g. adeno-tonsillar hypertrophy[10], chronic nasal congestion, allergic rhinitis) and that the tooth grinding and restlessness in bed observed were likely the consequence of fragmented sleep. In hindsight, these children should have been immediately referred to the paediatric sleep physician or ENT surgeon for further investigation.
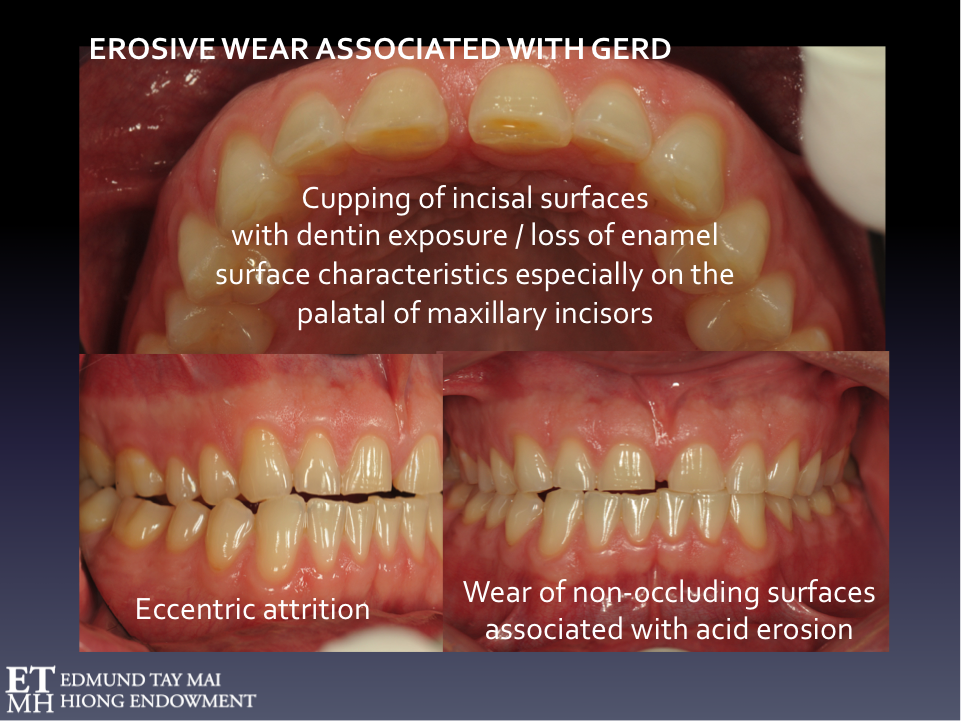
There’s also good evidence for SB being a protective arousal-related response to stimulate saliva secretion enabling the neutralization of oesophageal acid [3]. NREM sleep bruxers with comorbid OSA or UARS who also present with severe dental attrition combined with erosive wear should be screened for sleep-related gastro-esophageal reflux disorder (GERD) and laryngo-pharyngeal reflux (LPR) a.k.a. respiratory or ‘silent’ reflux, as the latter is more common in these patients because of the associated increase in negative intrathoracic pressure. The LPR patient more often present with symptoms such as hoarseness, chronic cough, post-nasal drip and frequent throat clearing unlike GERD patients who typically complain of heartburn and indigestion. They would benefit more from a referral to an ENT rather than the gastroenterologist[11]. If confirmed, these patients may need proton pump inhibitors e.g. Omeprazole, a strict low-acid diet, and alkaline water (pH of 8.8 to inactivate pepsin) in addition to protective occlusal appliances worn during sleep. (Fig. 4) It is ironic that the conventional full occlusal coverage flat plane ‘stabilization splints’ we were all taught in Dental School to fabricate for bruxism are now contraindicated in patients with sleep disordered breathing[12] as they have been shown to actually worsen apnea-hyponea index (AHI) and respiratory disturbance index (RDI)!
Fig. 4 Phasic NREM SLEEP BRUXISM with comorbid UARS and GERD
REM Sleep Bruxism
Although ‘generic’ RMMA-SB episodes are observed mostly in NREM stage 1 and 2, sleep stage shifts and in the transition period from NREM to REM sleep, a ‘sinister’ 10% occur in REM [13]. Fig. 5 REM SB has a entirely different etiopathology from NREM SB and is possibly a subclinical manifestation of REM sleep behavior disorder (RBD) [14] i.e. a ‘Parasomnia’ (Category 5: International classification of sleep disorders 3rd Edition, AASM 2014) [15].
RMMA-SB occuring during REM has special significance because of the general attenuation of protective reflexes during this phase of sleep. Patients reporting severe destructive bruxism were found to have more RMMA-SB episodes and more bruxing seconds per min of REM sleep [16]. Although the prevalence of REM sleep disorder behavior is about 0.6% of the general population, it is over-represented in the prosthodontic patient population. About 1 in 4 patients referred to me for prosthodontic full mouth reconstruction for severe dental attrition exhibited REM sleep bruxism. These were individuals who presented with significant morning symptomology (dental/restorative damage, tooth mobility, odontalgia, TMJ arthralgia, masticatory muscle myalgia, delayed onset muscle soreness and/or transient headache) as well as degenerative joint changes in the long term. REM SB has been referred to as “DESTRUCTIVE BRUXISM” [16]. Without nocturnal occlusal splint protection, I’ve seen too many properly osseointegrated implant-supported prostheses fail in these patients.
One of the reasons why Clonidine, an alpha 2-receptor agonist, is so effective in the treatment of severe sleep bruxism, especially the REM variant, may be because it significantly reduces the median percentage of time spent in REM sleep stage compared to Clonazepam and placebo [17].
The ongoing practice of only scoring RMMA frequency in PSG studies and not bothering to distinguish between episodes occurring in REM vs NREM remains a major setback. The Montreal group in 2007[18] had enormous difficulty explaining why the SB subgroup with low RMMA frequency (<4 episodes an hour) was associated with a higher risk of pain even though Ware & Rugh [16] had already pointed out as early as 1988 that ‘as little as 1 min of REM sleep bruxism per night may contribute to destructive bruxism symptom’. Ironically, the hypnogram of RMMA distribution in the various sleep stages in Fig. 5A published by the same group [19] could have easily demystified this otherwise puzzling association.
Interestingly, another more recent study [20] found 25% of patients with REM sleep behavior disorder met this PSG criteria for low RMMA frequency. We, therefore, should always rule out secondary bruxism and recommend further neurologic assessment whenever we encounter REM SB in our patients especially when they also report a familial history of Parkinson disease or other synucleinopathies.
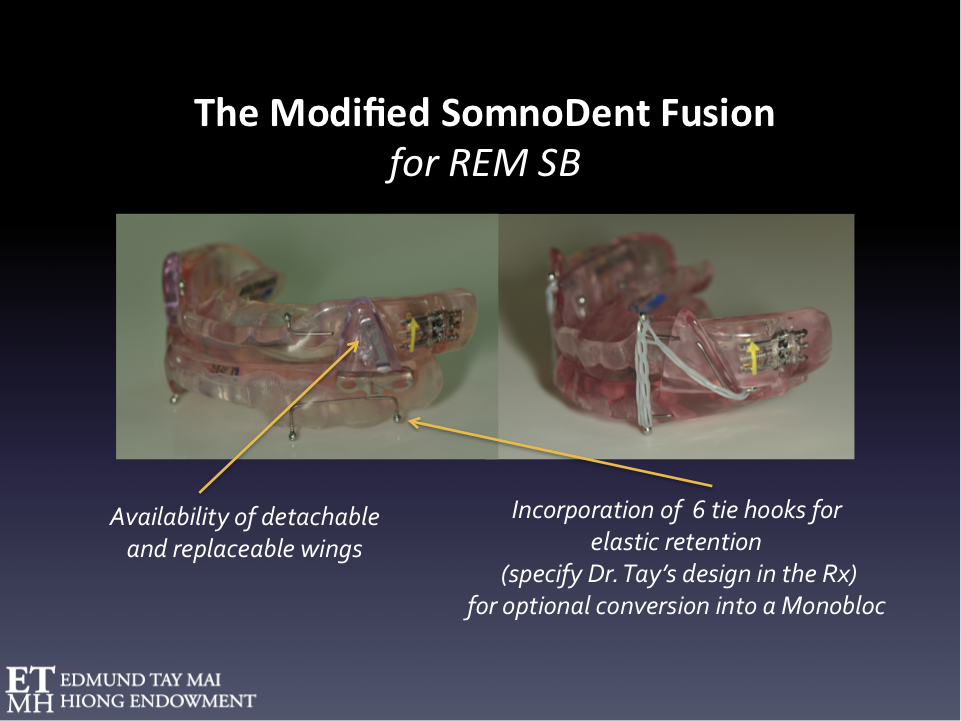
Fig. 5 Orthotic therapy for REM SB
The modified SomnoDent Fusion is a Mandibular Advancement Splint (MAS) that could be considered in the management of SB when there is comorbid SDB. It is ideal in the management of these ‘destructive’ REM sleep bruxers as the lateral wings are metal reinforced and replaceable. Tying the maxillary and mandibular plates together with orthodontic power chains prevents injurious mandibular torqueing that not uncommonly occur during REM SB episodes.
Conventional PSG only monitors superficial masseter and temporalis EMG activity but the resultant mandibular movement and the recurrent microtrauma-producing mandibular torquing that sometimes occur (and are very difficult to document if the overnight study was performed without video recording) during these REM bruxism episodes are influenced by varied vector combinations of all the contracting elevator and depressor muscles, including the medial and lateral pterygoids. I have had cases where the torquing (presumably during REM) was so extreme that the coronoid process of the mandible was repeatedly wrenched against the buccal aspects of the maxillary tuberosity of the patient causing unusually sited mucosal ulcers - the patient was not able to reproduce this parasomniac range of movement whilst awake!
I’m sure you also have come across patients with very severe attrition but report no pain or other morning symptoms – these individuals not uncommonly present with complete denture-inspired Balanced Occlusion schemes which do a great job in preventing mandibular torquing. Mandibular advancement splint designs used in the management of sleep disordered breathing [21] are especially effective in reducing painful waking symptoms in patients with a low frequency of RMMA especially when the upper and lower plates are tied together as they not only presumably open the upper airway (thereby reducing the potential for secondary NREM SB) but also prevent this violent torquing of the mandible during REM SB episodes. Tying the maxillary and mandibular plates together effectively converts potentially injurious involuntary eccentric muscle contraction (associated with delayed onset muscle soreness), during a phase of sleep where there are no protective reflexes, into less damaging isometric contractions when the muscles are held at a fixed length. (Fig. 6) Patients would often tell us that these orthotic appliances have ‘stopped their bruxism’ but all they actually did was alleviate morning symptomology. The PSG has been invaluable in dispelling notions harbored by some dentists that their peripheral interventions could address a complex, centrally-regulated brainstem oromotor phenomenon or that patients with sleep bruxism had a rare endogenous lack of poly(methyl methacrylate)!
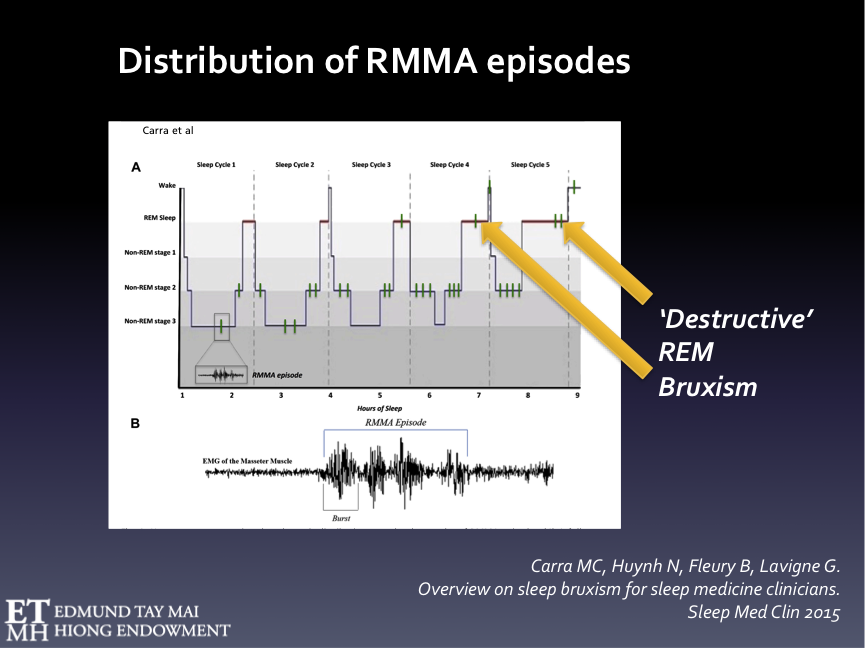
Fig. 6A Hypnogram representing the schematic distribution of RMMA episodes over the sleep cycles.
RMMA episodes are more frequently observed during NREM stage 1 & 2, sleep stage shift, and pre-REM sleep periods. RMMA episodes in REM stage occur less frequently. The latter, however, have greater potential to cause stomatognathic damage and we’ve had patients who only exhibited REM SB without the NREM variety.
Fig. 6B Example of an RMMA episode
Defined on the masseter electromyographic channel as an activity of at least 3 consecutive EMG bursts (frequency 1Hz) lasting greater or equal to 0.25 sec.
Although NREM SB nearly always occurs as a consequence of arousal, we have documented a reversed temporal relationship between arousal and REM RMMA-SB event in certain patients i.e. REM RMMA-SB can cause REM arousals! It has also been our experience that Botulinum toxin type A (Botox) injections into the superficial masseter & temporalis muscles would stop the frequency of these REM arousals for up to 6 months in some patients and should be considered in patients with elevator muscle hypertrophy, severe stomatognathic symptomology and/or associated violent REM arousals. Botox reduces the amplitude but not the frequency of these RMMA episodes [22].
References
Macaluso, G.M., et al., Sleep bruxism is a disorder related to periodic arousals during sleep. Journal of Dental Research,1998. 77:565-573.
Carra, M., et al., Sleep bruxism and sleep arousal: an experimental challenge to assess the role of cyclic alternating pattern. Journal of Oral Rehabilitation, 2011. 38(9): p. 635-642.
Miyawaki, S., et al., Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep, 2003. 26(4): p. 461-465.
Khoury, S., et al., A significant increase in breathing amplitude precedes sleep bruxism. Chest, 2008. 134(2): p. 332-337.
Simmons, J.H., Neurology of sleep and sleep-related breathing disorders and their relationships to sleep bruxism. Journal of the California Dental Association, 2012. 40(2): p. 159-167.
Tan M.W.Y., et al., Prevalence of sleep bruxism and its association with obstructive sleep apnea in adult patients: A retrospective polysomnographic investigation. Journal of Oral & Facial Pain and Headache, 2018. 33(3)::269-277.
Carra, M.C., et al., Sleep bruxism, snoring, and headaches in adolescents: short-term effects of a mandibular advancement appliance. Sleep Medicine, 2013. 14(7): p. 656-661.
Oksenberg, A. and E. Arons, Sleep bruxism related to obstructive sleep apnea: the effect of continuous positive airway pressure. Sleep Medicine, 2002. 3(6): p. 513-515.
Sjöholm, T., et al., Sleep bruxism in patients with sleep-disordered breathing. Archives of Oral Biology, 2000. 45(10): p. 889-896.
DiFrancesco, R.C., et al., Improvement of bruxism after T & A surgery. International Journal of Pediatric Otorhinolaryngology, 2004. 68(4): p. 441-445.
Koufman JA., Low-acid diet for recalcitrant laryngopharyngeal reflux: therapeutic benefits and their implications. Ann Otol Rhinol Laryngol. 2011. 120:281-287.
Gagnon, Y., et al., Aggravation of respiratory disturbances by the use of an occlusal splint in apneic patients: a pilot study. International Journal of Prosthodontics, 2004. 17(4).
Carra, M.C., N. Huynh, and G. Lavigne, Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dental Clinics, 2012. 56(2): p. 387-413.
Tachibana, N., et al., Sleep bruxism as a manifestation of subclinical rapid eye movement sleep behavior disorder. Sleep, 1994. 17(6): p. 555-558.
Medicine, A.A.o.S., International classification of sleep disorders–third edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine, 2014.
Ware, J.C. and J.D. Rugh., Destructive bruxism: sleep stage relationship. Sleep, 1988. 11(2): p. 172-181.
Sakai T, KatoT, Yoshizawa S, et al., Effect of clonazepam and clonidine on primary sleep bruxism: a double blind, crossover, placebo-controlled trial. Journal of Sleep Research, 2017. 26: p.73-83.
Rompré, P., et al., Identification of a sleep bruxism subgroup with a higher risk of pain. Journal of dental research, 2007. 86(9): p. 837-842.
Carra, M.C., et al., Overview on sleep bruxism for sleep medicine clinicians. Sleep Medicine Clinics, 2015. 10(3): p. 375-384.
Abe S, Gagnon J-F, Montplaisir J Y, Postuma RB, Rompre P, Huynh NT, Kato T, Kawano F, Lavigne GJ. Sleep bruxism and oromandibular myoclonus in rapid eye movement sleep behavior disorder: a preliminary report. Sleep Med, 2013. 10: p.1024-30.
Franco, L., et al., A mandibular advancement appliance reduces pain and rhythmic masticatory muscle activity in patients with morning headache. Journal of Orofacial Pain, 2011. 25(3): p. 240.
Shim, Y.J., et al., Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: a polysomnographic evaluation. Journal of Clinical Sleep Medicine, 2014. 10(3): p. 291.
The views and opinions expressed in this article are those of the author's alone.